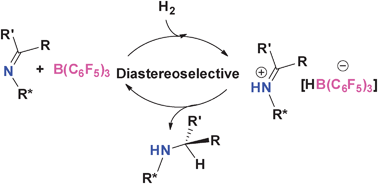
Reductions of chiral ketimines effected under H2 by catalytic amounts of B(C6F5)3 result in moderate to excellent diastereoselectivities. In the case of camphor and menthone derived imines, the reductions proceeded with greater than 95% diastereoselectivity.