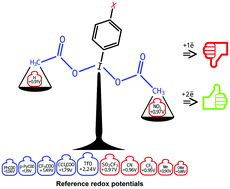
Computational prediction of the oxidative ability of hypervalent iodine reagents has been performed based on redox potentials, calculated at the B3LYP/6-311+G(d,p) level of theory with the SDD ECP46MDF pseudopotential and its adapted basis set for the iodine atom. It was shown that the main factor determining the power of Ar-I(L)2 as an oxidative reagent is the nature of the ligands at the iodine atom. The presence of strong electron-withdrawing substituents in the aromatic ring, as well as the use of a highly polar solvent, provides an increase in the oxidative ability of Ar-I(L)2.