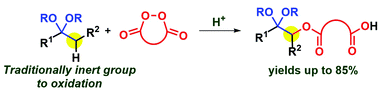
An acetal fragment is a well-known protective group which does not activate the neighboring α-position. Selective functionalization of the non-activated acetal α-position with formal retaining of the acetal fragment was realized using cyclic diacyl peroxides. The discovered oxidative C–O coupling of cyclic diacyl peroxides with acetals leads to α-acyloxy acetals with a free carboxylic acid group in 42–85% yields. The reaction probably proceeds via in situ enol ether formation, oxidative [5 + 2] cycloaddition, and the recovery of the acetal fragment.