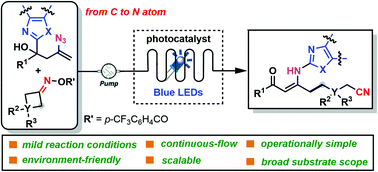
A photoinduced 1,4-heteroaryl migration from a carbon center to a nitrogen center accompanied by a cyanoalkylacylation of heterocyclic-substituted azidyl homoallylic alcohols and cycloketone oxime esters has been described. This simple and powerful protocol merged the dual C–C bond cleavage with C–N bond formation, providing an efficient and environmentally safe approach to a variety of synthetically useful cyanoalkyl-containing β-enamino ketones with outstanding selectivity and commendable functional group compatibility. Moreover, the application of microflow technique enhanced these reactions compared with the equivalent batch reaction, significantly reducing the reaction times to 5–9.3 min and broadening the substrate scope to more than 35 successful substrates.