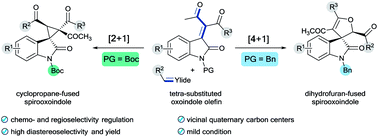
Protecting group-controlled annulations of tetra-substituted oxindole olefins and sulfur ylides have been achieved for the synthesis of multifunctional cyclopropane- and dihydrofuran-fused spirooxindoles. Under precise annulation regulation, a variety of cyclopropane- and dihydrofuran-fused spirooxindoles containing vicinal quaternary carbon centers were produced in up to 90% yield with up to 20 : 1 dr. This reaction demonstrates high regio-, chemo- and diastereoselectivity, broad functional group tolerance and gram-scale capacity.