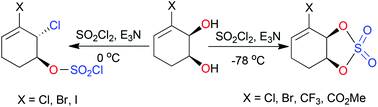
Monocyclic allylic cis-1,2-diols reacted with sulfuryl chloride at 0 °C in a regio- and stereo-selective manner to give 2-chloro-1-sulfochloridates, which were hydrolysed to yield the corresponding trans-1,2-chlorohydrins. At −78 °C, with very slow addition of sulfuryl chloride, cyclic sulfates were formed in good yields, proved to be very reactive with nucleophiles and rapidly decomposed on attempted storage. Reaction of a cyclic sulfate with sodium azide yielded a trans-azidohydrin without evidence of allylic rearrangement occurring. An enantiopure bicyclic cis-1,2-diol reacted with sulfuryl chloride to give, exclusively, a trans-1,2-dichloride enantiomer with retention of configuration at the benzylic centre and inversion at the non-benzylic centre; a mechanism is presented to rationalise the observation.