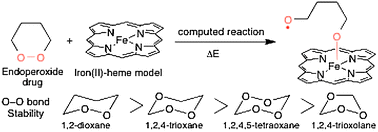
Several endoperoxide compounds are very efficient antimalarial analogues of the natural drug artemisinin. Quantum chemical calculations have been used to correlate the computed free energies of the O–O bond with respect to the total number of oxygen atoms contained in the cycle, and with the size/strain of the cycle (5- or 6-membered cycles). The gas-phase homolysis of the O–O bond has been studied for five- and six-membered oxygenated cycles which are models of the “real” drugs. Our results indicate that, in 6-membered cycles, the stability order is the following: 1,2-dioxane > 1,2,4-trioxane > 1,2,4,5-tetraoxane. In cycles containing 3 oxygen atoms, the 5-membered cycle 1,2,4-trioxolane was found much less stable than its 6-membered counterpart 1,2,4-trioxane. This feature indicates the possible role of the cycle strain for the O–O bond stability, and may also explain the high antimalarial activity of some trioxolane derivatives. Similar trends in the O–O bond strength have been found for the real antimalarial drugs. However, the O–O bond stability is not in itself a decisive argument to anticipate the antimalarial activity of drugs.