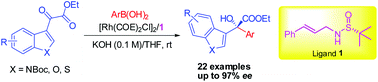The first example of catalytic asymmetric 1,2-addition of arylboronic acids to heteroaryl α-ketoesters has been developed for the highly efficient and enantioselective synthesis of quaternary carbon-containing heteroaromatic α-hydroxy esters. The reaction works well with a variety of α-ketoesters including 3-indoleglyoxylates, 3-benzofuranglyoxylates and 3-benzothiopheneglyoxylates under very mild conditions, affording the corresponding products in moderate to good yields with high enantiomeric excesses (up to 97%).