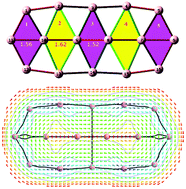
We analyze the thermodynamic stability of some small elongated boron clusters and confirm the relationship between their planarity and their inherent electron configuration […σ2(n+1) π12(n+1) π22n]. Delocalized σ electrons in an elongated bare boron cluster and 2c-2e C–H bonds in a corresponding elongated hydrocarbon play a vital role in maintaining their planar structure. Through the eigenstates derived from a model of a particle moving in a rectangle, the rectangle model, our study suggests that the larger planar elongated boron clusters are not thermodynamically stable. A partition of the electron densities which is consistent with the electron count, points out that the dianionic, neutral and dicationic B102−/0/2+ clusters are doubly σ and π aromatic, singly π aromatic, and doubly σ and π antiaromatic, respectively.