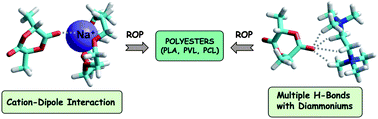
Quaternary ammoniums associated with bis(trifluoromethane)sulfonimide (NTf2) or tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BARF) counterions were readily prepared from commercially available tertiary amine, amidine, guanidine and pyridine. As predicted by molecular modelling, these ammonium salts proved to be efficient multiple H-bond donor catalysts in the ring-opening polymerization of cyclic esters (lactide, δ-valerolactone and ε-caprolactone). In addition, a sodium(I) complex of [15-c-5] crown-ether paired with NTf2 or BARF was shown to activate the cyclic monomers through a cation–dipole interaction. These simple and non-protonated ionic structures appeared as attractive alternative organocatalysts to classical H-bond donors for the activation of carbonyl bonds.