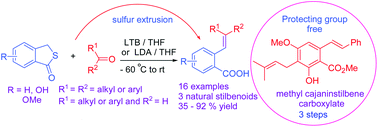
The synthesis of stilbenoids and styryl carboxylic acids is accomplished with high E-stereoselectivity by olefination of aldehydes with thiophthalides under basic conditions. The olefination is highly atom-efficient as it only loses elemental sulfur during the reaction. This olefination, in conjunction with retro Kolbe–Schmitt reaction, allows facile synthesis of E-hydroxystilbenoids with minimal employment of protecting groups. This study also discloses two important findings: formation of i) 4-methylsulfanyl isocoumarins and ii) an 2-arylindenone.