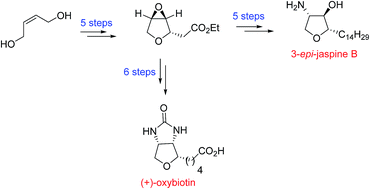
A new synthesis of cytotoxic anhydrophytosphingosine 3-epi-jaspine B (34.7% overall yield; 97% ee) and (+)-oxybiotin (21.2% overall yield; 97% ee), bioactive oxygen analogue of biotin, is described starting from commercially available cis-2-butene-1,4-diol. The key reactions employed in the synthesis include Sharpless asymmetric epoxidation and a novel tandem desilylation oxa-Michael addition reaction strategy to construct a tetrahydrofuran core (dr >99%).