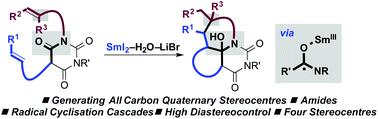
Radical–radical cyclisation cascades, triggered by single-electron-transfer to amide-type carbonyls using SmI2–H2O–LiBr, result in the selective construction of quaternary carbon stereocentres. The cascades deliver tricyclic barbiturates with four stereocentres in good yield and with excellent diastereocontrol.