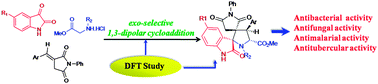
A series of spiro[pyrrolidin-2,3′-oxindoles] has been synthesized by exo-selective 1,3-dipolar cycloaddition reaction of a stabilized azomethine ylide, generated in situ by thermal [1,5]-prototropy, across various (E)-3-arylidene-1-phenyl-pyrrolidine-2,5-diones. The stereochemistry of these N-heterocycles has been confirmed using an X-ray diffraction study. To rationalize the observed regio- and stereoselectivity, DFT calculations at the B3LYP/6-31G(d,p) level were employed. It was found that this reaction preferentially affords the kinetic product. The compounds have been screened for their in vitro antibacterial, antifungal, antimalarial and antitubercular activities. Several compounds exhibited good activities comparable to those of established standard drugs.