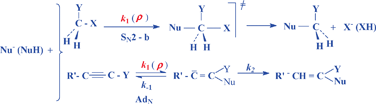
Changes of the activation parameters, ΔH‡ and ΔS‡, in the SN2, SNV, AdN, SNAr and acyl-transfer reactions with phenol, aniline and pyridine nucleophiles in various solvents were correlated with σ constants of the substituents in the aromatic ring of the nucleophiles. The resultant δΔH‡ and δΔS‡ reaction constants are linearly related for variations of substituents at the nucleophile. Correlation of δΔH‡vs. δΔS‡ allow the estimation of the contribution of changes of the internal enthalpy, δΔH‡int, to the enthalpy reaction constant, δΔH‡, which gives a single linear dependence on the Hammett ρ reaction constants for all bimolecular nucleophilic reactions. The deviations from dependence of δΔH‡intvs. ρ can be interpreted in terms of changes of the transition state structure or reaction mechanism. The results obtained show that the substituent effects in the nucleophiles on the charge development in the transition state are governed by the magnitude of δΔH‡int.