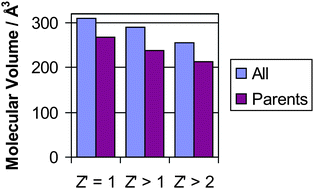
Systematic analysis of the Cambridge Structural Database (CSD) using parameters based on the shape, size and “awkwardness” of organic molecules shows that compounds which crystallise with Z′ > 1 are in general smaller (by around 50 Å3 on average) and less flexible (with ca. two fewer rotatable bonds) than molecules which crystallise with Z′ = 1. Molecules which are known to form co-crystals are, on average, even smaller and less flexible compared to molecules which crystallise with Z′ = 1. Thus formation of co-crystals or structures with Z′ > 1 is strongly linked to small, rigid, awkwardly shaped molecules that have more constraints on their crystal packing requirements. These results have some predictive utility in determining the likelihood of hydrate formation in pharmaceuticals, for example.