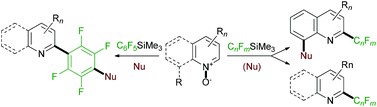
The scope and mechanistic implications of the direct transformation of heterocyclic N-oxides to 2-trifluoromethyl-, and related perfluoroalkyl- and perfluoroaryl-substituted N-heterocycles has been studied. The reaction is effected by perfluoroalkyl- and perfluorophenyltrimethylsilane in the presence of strong base. In situ displacement of the para-fluoro substituent in the pentafluorophenyl ring and the methoxy group in 8-methoxyquinolines with additional nucleophiles allows for further site-selective refunctionalization of the N-heterocyclic products.