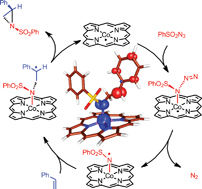
The mechanism of cobalt(II) porphyrin-mediated aziridination of styrene with PhSO2N3 was studied by means of DFT calculations. The computations clearly indicate the involvement of a cobalt ‘nitrene radical’ intermediate in the CoII(por)-catalyzed alkene aziridination. The addition of styrene to this species proceeds in a stepwise fashion via radical addition of the ‘nitrene radical’ C to the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bond of styrene to form a γ-alkyl radical intermediate D. The thus formed tri-radical species D easily collapses in an almost barrierless ring closure reaction (TS3) to form the aziridine, thereby regenerating the cobalt(II) porphyrin catalyst. The radical addition of the ‘nitrene radical’ C to the olefin (TS2) proceeds with a comparable barrier as its formation (TS1), thus providing a good explanation for the first order kinetics in both substrates and the catalyst observed experimentally. Formation of C is clearly accelerated by stabilization of C and TS1viahydrogen bonding between the S
C double bond of styrene to form a γ-alkyl radical intermediate D. The thus formed tri-radical species D easily collapses in an almost barrierless ring closure reaction (TS3) to form the aziridine, thereby regenerating the cobalt(II) porphyrin catalyst. The radical addition of the ‘nitrene radical’ C to the olefin (TS2) proceeds with a comparable barrier as its formation (TS1), thus providing a good explanation for the first order kinetics in both substrates and the catalyst observed experimentally. Formation of C is clearly accelerated by stabilization of C and TS1viahydrogen bonding between the S![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O and N–H units. The computed radical-type mechanism agrees well with all available mechanistic and kinetic information. The computed free energy profile readily explains the superior performance of the CoII(porAmide) system with H-bond donor functionalities over the non-functionalized Co(TPP).
O and N–H units. The computed radical-type mechanism agrees well with all available mechanistic and kinetic information. The computed free energy profile readily explains the superior performance of the CoII(porAmide) system with H-bond donor functionalities over the non-functionalized Co(TPP).
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bond of
C double bond of ![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O and N–H units. The computed radical-type mechanism agrees well with all available mechanistic and kinetic information. The computed free energy profile readily explains the superior performance of the CoII(porAmide) system with H-bond donor functionalities over the non-functionalized Co(TPP).
O and N–H units. The computed radical-type mechanism agrees well with all available mechanistic and kinetic information. The computed free energy profile readily explains the superior performance of the CoII(porAmide) system with H-bond donor functionalities over the non-functionalized Co(TPP).