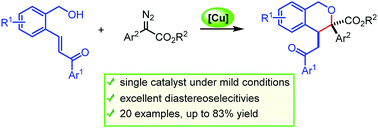
A highly diastereoselective approach for the rapid construction of an isochroman skeleton was achieved by the copper(II)-catalyzed transformation of alcohol-tethered enones and diazo compounds. This transformation was proposed to proceed through the intramolecular Michael-type trapping of an in situ generated oxonium ylide intermediate. The copper(II) catalyst may play a dual role in catalyzing diazo decomposition as well as activating the enone unit. With this method, a series of 3,4-substituted isochromans were obtained with excellent diastereoselectivities under very mild reaction conditions.