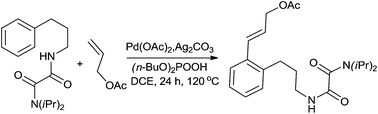
A protocol for palladium-catalyzed ortho C(sp2)–H alkenylation via a rarely reported seven-membered palladacycle with oxalyl amide as a directing group was reported. The range of olefins was the broadest yet reported for this kind of C–H alkenylation reaction. For example, allyl acetate, allyl alcohol derivatives, acraldehyde, acrylate and acrylonitrile were all tolerated in this C–H transformation. Sequential alkenylation reactions were also achieved to construct the complex olefinated arenes in good yields in one pot.