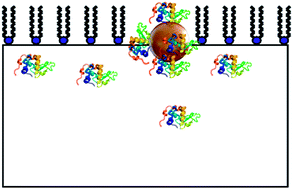
Binding to colloidal structures such as gold nanoparticles (Aunps) is known to affect protein conformation, potentially altering the function and bioinvasiveness of the biopolymer. As the use of protein–Aunp constructs is widespread and increasing, an improved understanding of the invasiveness of nanomaterials is of fundamental interest and crucial for the burgeoning fields of nanotherapy and nanotoxicology. Here, we report on the interaction of bovine α-lactalbumin (BLA) modified 30 nm Aunps with neutral, negatively charged and mixed neutral : negative phospholipid Langmuir monolayers at pH 7.4, where little or no interaction has been reported for native BLA. The resulting equilibria and film properties were studied using surface pressure–area isotherms. Interaction of BLA with Aunps was investigated using UV-vis and fluorescence (steady-state and time-resolved, using intrinsic and extrinsic probes) spectroscopies. We find that upon binding to the Aunps, BLA partially unfolds, which in turn imparts higher interfacial activity and a stronger affinity towards phospholipid monolayers compared to the native protein.