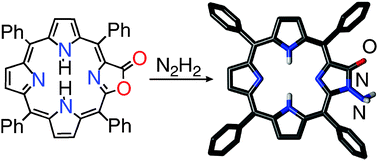
Reaction of meso-tetraphenylporpholactone with hydrazine converts the lactone moiety to an N-aminolactam. It also reduces the opposite pyrrolic moiety of both the starting material and the N-aminolactam, generating chlorin-like chlorolactone and N-aminochlorolactam, respectively. Reductive N–N cleavage of the N-aminoporpholactam generates the parent porpholactam.