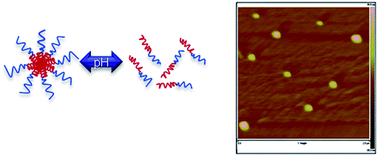
A series of pH responsive synthetic polypeptides has been developed based on an N-carboxyanhydride ring opening polymerization combined with a facile and versatile click chemistry. Poly(γ-propargyl L-glutamate) (PPLG) homopolymers and poly(ethylene glycol-b-γ-propargyl L-glutamate) (PEG-b-PPLG) block copolymers were substituted with various amine moieties that range in pKa and hydrophobicity, providing the basis for a library of new synthetic structures that can be tuned for specific interactions and responsive behaviors. These amine-functionalized polypeptides have the ability to change solubility, or reversibly self-assemble into micelles with changes in the degree of ionization; they also adopt an α-helical structure at biologically relevant pHs. Here we characterize the pH responsive behavior of the new polypeptides and the hydrolysis of the ester containing amine side chains. We examine the reversible micellization with block copolymers of the polypeptides and nucleic acid encapsulation that demonstrate the potential use of these materials for systemic drug and gene delivery.