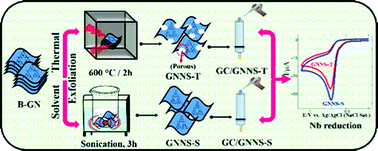
The present investigation reports the preparation of graphitic carbon nitride nanosheets (GNNS) from bulk GN (B-GN) by liquid and thermal exfoliation methods and compares their electrocatalytic activity towards the reduction of nitrobenzene (Nb). The SEM images of GNNS powder prepared by water exfoliation (GNNS-S) show the presence of thin sheets along with a few multilayers, whereas highly crumpled thin sheets containing small pores were noticed for GNNS prepared by thermal exfoliation (GNNS-T). The charge transfer resistance (RCT) values obtained from Nyquist plots were found to be 19.2, 21.9, 5.49 and 2.11 kΩ, respectively, for bare GC, B-GN, GNNS-T and GNNS-S modified GC electrodes indicating the lowest impedance of GNNS-S. Furthermore, the electroactive surface area (EAS) of the respective electrodes was estimated to be 0.062, 0.039, 0.108 and 0.123 cm2, revealing that the EAS of the GC/GNNS-S electrode was higher than that of GC/GNNS-T. The performance of the GNNS-S and GNNS-T modified electrodes was compared by studying the electrochemical reduction of Nb. In contrast to the GC/GNNS-T electrode, GC/GNNS-S enhanced the reduction current by 1.2-fold. The higher electrocatalytic activity of GC/GNNS-S was ascribed to its higher electronic conductivity and EAS than that of the GC/GNNS-T electrode. The Nb reduction current was increased linearly upon increasing its concentration from 0.5 to 2000 μM and the LOD was found to be 7.3 nM (S/N = 3). Finally, the practical application of the present sensor was established by determining Nb in tap water samples.