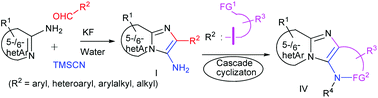
KF-mediated nucleophilic activation of TMSCN as a functional isonitrile equivalent establishes an efficient and chemoselective Ugi-type multicomponent reaction of a heterocyclic amidine and aldehyde with TMSCN in water. In this approach, the use of isocyanide is circumvented, known competing reactions are virtually eliminated, pure products are obtained by a non-chromatographic method, and therapeutically relevant and diverse N-fused 3-aminoimidazoles can be prepared from a wide variety of aldehydes and heterocyclic amidines. This reaction coupling with cascade cyclization provides various privileged tetracyclic heteroaromatic scaffolds.