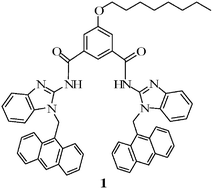
A simple anthracene-linked benzimidazole diamide, 1, has been designed and synthesized. 1 was found to undergo a subtle change in conformation, followed by destruction of the inherently present excimer in the presence of organic sulfonic acids and selective metal ions. Destruction of the excimer did not take place in the presence of carboxylic acids and even stronger trifluroacetic acid. This finding was taken as a possible tool for distinguishing organic sulfonic acids from carboxylic acids. A similar situation arose in the presence of Cu2+, Co2+ and Ni2+, among the other metal ions studied, and the cleft was selective for Cu2+. The binding interaction was followed using 1H NMR, UV-vis and fluorescence spectroscopic methods.