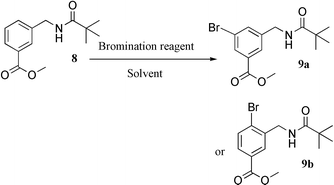
A variety of aromatic compounds with both activating and deactivating substituents were brominated with sodium monobromoisocyanurate (SMBI) 1, diethyl ether, diethyl ether–methanesulfonic acid, trifluoroacetic acid, or sulfuric acid were employed as solvents. Thus nitrobenzene was conveniently brominated in sulfuric acid, benzene was readily monobrominated in diethyl ether–methanesulfonic acid, and phenol was selectively brominated at the ortho position under mild conditions in refluxing diethyl ether. With substituents that are easily protonated, trifluoroacetic acid may be employed as solvent in the reaction with 1, in contrast NBS was ineffective in trifluoroacetic acid. This renders 1 a superior reagent relative to NBS. In addition to aromatics, alkenes, ketones and esters were also brominated with 1. Diethyl malonate was brominated with 1 and then subjected to a Bingel reaction with NaH to afford the desired methanofullerene in reasonable yield.