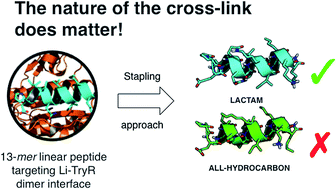
All-hydrocarbon and lactam-bridged staples linking amino acid side-chains have been used to stabilize the α-helical motif in short 13-mer peptides that target critical protein–protein interactions at the dimerization interface of Leishmania infantum trypanothione reductase (Li-TryR). The design of the best positions for covalent hydrocarbon closure relied on a theoretical prediction of the degree of helicity of the corresponding cyclic peptides in water. Selected (i, i + 4) and (i, i + 7) hydrocarbon-stapled peptides were prepared by using solid-phase synthesis protocols and optimized ring-closing metathesis reactions under microwave conditions. Structural analysis by NMR spectroscopy confirmed high helical contents in aqueous TFE solutions for both types of helix-constrained cyclic peptides. Remarkably, the ability to prevent Li-TryR dimerization was reduced in both (i, i + 4) and (i, i + 7) hydrocarbon stapled peptides but was retained in the corresponding (i, i + 4) Glu–Lys lactam-bridged analogue, which also showed a higher resistance to proteolytic degradation by proteinase K relative to the linear peptide prototype. In silico studies indicated that the introduction of a hydrocarbon staple vs. a lactam bridge likely perturbs critical interactions required for proper binding of the peptide to the Li-TryR monomer.