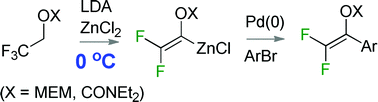
Difluoroalkenylzinc reagents prepared from 1-(2′-methoxy-ethoxymethoxy)-2,2,2-trifluoroethane and 1-(N,N-diethylcarbamoyloxy)-2,2,2-trifluoroethane at ice bath temperatures underwent Negishi coupling with a range of aryl halides in a convenient one pot procedure. While significant differences between the enol acetal and carbamate reagents were revealed, the Negishi protocol compared very favourably with alternative coupling procedures in terms of overall yields from trifluoroethanol.