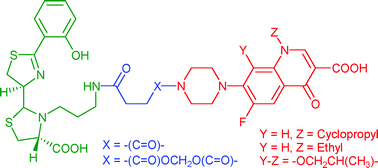
Pyochelin is a siderophore common to Pseudomonas aeruginosa and several other pathogenic bacteria. A pyochelin functionalized at the N3′′ position with a propyl-amine extension was previously synthesized. In the present work we proved that this analog binds FptA, the pyochelin outer membrane receptor, and transports iron(III) efficiently into bacteria. This functionalized pyochelin seemed to be a good candidate for antibiotic vectorization in the framework of a Trojan horse prodrug strategy. In this context, conjugates between pyochelin and three fluoroquinolones (norfloxacin, ciprofloxacin and N-desmethyl-ofloxacin) were synthesized with a spacer arm that was either stable or hydrolyzable in vivo. Some pyochelin–fluoroquinolone conjugates had antibacterial activities in growth inhibition experiments on several P. aeruginosa strains. However, these activities were weaker than those of the antibiotic alone. These properties appeared to be related to both the solubility and bioavailability of conjugates and to the stability of the spacer arm used.